The biomedical division of the Chrisey lab is located in the newly renovated J. Bennett Johnston Building, part of the Tulane University/Louisiana State University medical complex. During my PhD research, I worked on four main projects:
Through my work, I developed an intimate understanding of optomechanical systems - knowledge that is both beneficial for microscopy and laser-based cell/biomaterials processing. When the Chrisey Lab purchased its customized laser-based bioprinter, I had a hand in the design and implementation of the system. To see some of my bioprinting work, click on 3D bioprinting or Engineered tissue for breast cancer research.
Because light paths and laser beams are influenced by the same physics and optical components, it was natural for me to develop a strong interest for microscopy (and photography!). Here is a sampling of images I took for collaborative projects with other labs:
MDA-MB-231 breast cancer cells treated with MEK-inhibitor drug and stained for cytoskeleton (red) and nucleus (blue).
Tissue section containing a pre-invasive, cancerous prostrate gland.
Pancreatic cancer cells treated with anti-cancer drug and stained with crystal violet for cell morphology studies.
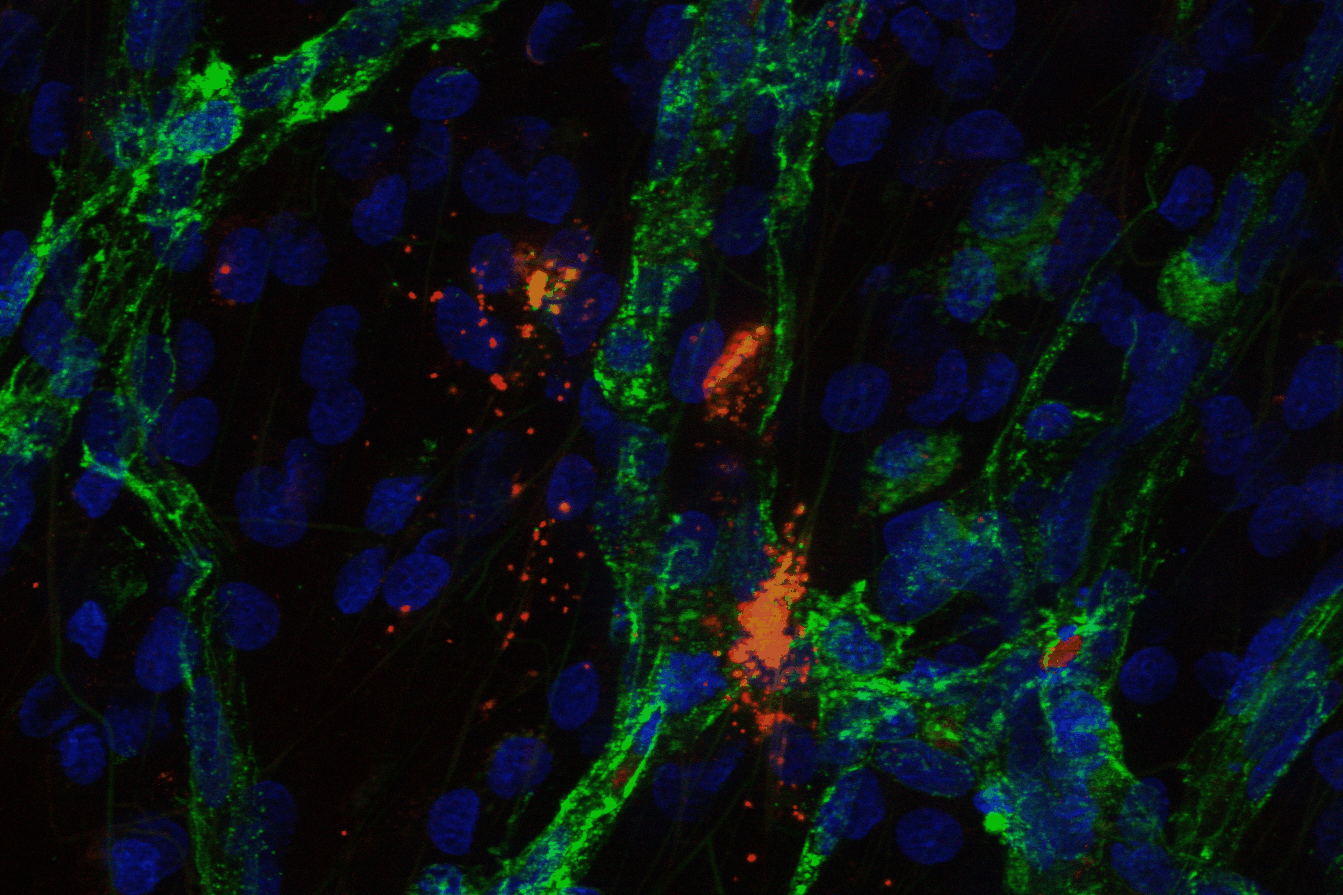
Cluster of MCF-7 breast cancer cells, some expressing GFP-conjugated vector protein.
As the former lab manager for the Biomedical Engineering Division in the Chrisey Lab, I maintained and troubleshooted equipment. Here are some of the facilities and equipment that I encountered on a daily basis:
Microscope
Description: Multi-modality epi-configuration microscope with temperature and CO2 control modules for live-cell timelapse imaging. Transmission light modes: bright field, phase contrast, differential interference contrast. Fluorescence filters: (Blue) Em = 390, Ex = 460; (Green) Em = 470, Ex = 525; (Red) Em = 565, Ex = 620 nm. Objective lens: 10x, 20x, 20xLD, 40x, 63x, 100x. Apotome module for confocal-like imaging.
Uses: (1) Multi-channel imaging in both transmission and wide-field fluorescent light, (2) Apotome imaging for optical slices, (3) mosaic imaging over large areas, (4) z-stack imaging of tissue samples, (5) live-cell timelapse imaging
Laser-based bioprinter
Description: Customized excimer UV laser system (ArF, 193 nm, near-Gaussian energy distribution, pulse width = ~8 ns, avg power = 5W @ 500 Hz) for micron-scale materials processing. System is integrated with photometer for real-time energy readings and motorized iris for beam sizing. Independently-controlled motorized ribbon and substrate stages allow for computer-aided design/manufacturing feasibility. System also includes in situ camera for real-time video feed at laser focus and incubator for temperature and humidity control in working space.
Uses: (1) Patterning of cells, (2) printing of single microbeads, (3) micromachining on metal or hydrogel materials
Cell culture room
Description: Three BSL2 laminar flow hoods for cell culture work. Each hood is equipped with up-to-date HEPA filters, UV sterilization, and in-line aspirators. The shared facility also houses cell culture supplies, 10 incubators, 2 centrifuges, and 2 light microscopes.
Uses: Cell culture work and sample preparation.